Synonyms
3,3-Dimethoxy-1-propene;3,3-Dimethoxypropene;3,3Dimethoxypropene;3,3Dimethoxy1propene
Molecular Formula
C5H10O2
Appearance
Colorless liquid
General Description
Acrolein dimethyl acetal is an acetal compound. As an intermediate, it's a highly valuable building block in pharmaceutical synthesis. Compared to acrolein, it offers superior stability, also it can efficiently introduce a three-carbon or derivatizable unit into complex molecular frameworks. Beyond pharmaceutical applications, its use is also expanding in the synthesis of fragrances, agrochemicals, and others.
Mechanism of Action
Acrolein dimethyl acetal is a synthetic intermediate with the highly reactive aldehyde group protected as an acetal. The acetal group is significantly more stable than an aldehyde group in basic and nucleophilic reagents as well as oxidizing agents. The acetal can also be cleaved reversibly to form the free aldehyde when desired. The terminal alkene functionality can be used as a nucleophile or as a Michael donor in further functionalization steps.
Application
The most common application of acrolein dimethyl acetal is in chemical synthesis, such as in the production of 3-pyrroline.
It is used to manufacture a variety of different chemicals, including cinnamaldehyde.
This chemical is employed in the pharmaceutical industry.
It is also utilized as a polymer in products such as coatings, varnishes, and surgical adhesives.
Acrolein dimethyl acetal is further applied as a plasticizer in the manufacture of nitrocellulose and other polymers.
Research reports that zinc-modified Hβ zeolite is catalytically active in the gas-phase aza-heterocyclic aromatization of acrolein dimethyl acetal with aniline for the synthesis of quinoline. The findings revealed that the Zn/Hβ catalyst displayed a higher selectivity to quinoline in comparison with the parent Hβ zeolite. Moreover, a possible reaction pathway for the formation of quinoline from acrolein dimethyl acetal and aniline in this gas-phase reaction system was also proposed.
 Fig. 1 Zn-promoted Hβ zeolite catalyzed acrolein dimethyl acetal and aniline to quionlines. (An Li.; et al. 2020)
Fig. 1 Zn-promoted Hβ zeolite catalyzed acrolein dimethyl acetal and aniline to quionlines. (An Li.; et al. 2020)
References
- An Li, et al. Zn-promoted Hβ zeolite for gas-phase catalyzed aza-heterocyclic-aromatization of acrolein dimethyl acetal and aniline to quinolines.Molecular Catalysis, 2020 May;486;110833.
3-Picoline is a chemical intermediate that has application as a building block for pesticides, fodder, and pharmaceuticals. The classical method of synthesizing 3‑methylpyridine from formaldehyde, acetaldehyde, and ammonia results in only very low yields. In contrast, the reaction between acrolein and ammonia significantly increases the yield to 30–60%. However, the equipment rapidly becomes clogged, and therefore, the reaction must be terminated soon after initiation. In addition, the materials are inherently unstable and toxic. To overcome these disadvantages, the synthesis of 3‑methylpyridine was performed via acrolein dimethyl acetal or acrolein diethyl acetal with ammonia. Acrolein dimethyl acetal or acrolein diethyl acetal, in the presence of acid catalysts, undergoes facile hydrolysis to acrolein and methanol or ethanol. This averts the polymerization which had previously occurred.
 Fig. 2 The formation of 3-picoline from acrolein dimethyl acetal and ammonia. (Luo, Cai-Wu.; et al. 2015)
Fig. 2 The formation of 3-picoline from acrolein dimethyl acetal and ammonia. (Luo, Cai-Wu.; et al. 2015)
References
- Luo, Cai-Wu, et al. Unsaturated aldehydes: a novel route for the synthesis of pyridine and 3-picoline. RSC Advances, 2015,67(5), 54090-54101.
What are the safety precautions for Acrolein Dimethyl Acetal?
Acrolein dimethyl acetal is classified under GHS H225 as a highly flammable liquid and vapor, presenting significant fire and explosion hazards.
What are the application scenarios of Acrolein Dimethyl Acetal?
Pharmaceuticals, pesticides, and fragrances industries.
Can I request acrolein dimethyl acetal samples?
Contact us to discuss sample availability of Procaine.
What is your standard delivery time for products?
Our product lead times are order-specific, but generally 2-4 weeks.

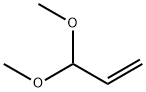
 Fig. 1 Zn-promoted Hβ zeolite catalyzed acrolein dimethyl acetal and aniline to quionlines. (An Li.; et al. 2020)
Fig. 1 Zn-promoted Hβ zeolite catalyzed acrolein dimethyl acetal and aniline to quionlines. (An Li.; et al. 2020)
 Fig. 2 The formation of 3-picoline from acrolein dimethyl acetal and ammonia. (Luo, Cai-Wu.; et al. 2015)
Fig. 2 The formation of 3-picoline from acrolein dimethyl acetal and ammonia. (Luo, Cai-Wu.; et al. 2015)


![Tert-Butyl 3-oxo-6-fluoro-2,3-dihydrospiro[indene-1,4'-piperidine]-1'-carboxylate](https://www.protheragen.ai/wp-content/themes/protheragen-ing/img/910442-55-6.png)










